What is semiconductor?
Before we delve into that, let's remember that there are four states of matter:
- solid
- gas
- liquid
- plasma
Solid materials can be divided into three categories based on the arrangement of their atoms or molecules:
1- Crystalline: Atoms are arranged periodically such as Silicon;

image-1
iron sheet shows the grains in different color

image-2
shows the different in atoms arrangement in three solid categories
As mentioned earlier, a crystal is made up of atoms arranged in a repeating pattern. If we imagine these atoms as solid spheres regularly spread out in space, this symmetric array of point in space called lattice Within this lattice, there's a volume or cell can be observed contains a number of atoms and has specific geometric shape like cube or pentagonal prism and repeated regularly forming the crystal this cell called unit cell.
Silicon unit cell called diamond unit cell thi name came from fact that the arrangement of atoms in diamond and silicon is same and consist 8 atoms as shown in image-3
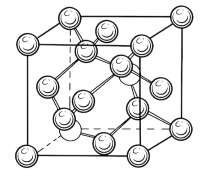
image-3
diamond unit cell
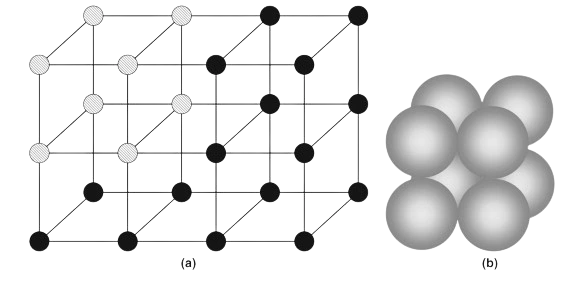
image-4
lattice and one unit cell
_ Semiconductors are group of materials having electrical conductivity intermediate between metals and insulators, it is categorized under solid crystalline material. we can control its conductivity by changing in temperature, optical excitation or by adding impurities. Semiconductors are found in column (IV) and neighboring columns of the periodic table. One of the most important characteristic of semiconductor material is band gap this property distinguishes it from metals and insulator.
But what we mean by band and gap?
_ Band mean a wide range and gap to illustrate that there is no electron can found in this region
To understand that, firstly let us remember that in individual atom the most outer filled or partially filled orbital called valence orbital and after that empty orbitals is called conduction orbital and between of them there is forbidden region where there is no any electron can be found, secondly we should take a simple model; when two identical atom come together to form solid various interactions occur lead to splitting of energy level each level will contain one electron.
The reason of splitting is being in the Pauli exclusion principle which state that no two electrons in given interacting system may have the same four quantum numbers, or simply no two electrons can be in same orbital and have the same spinning orientation in same physical system.
But if we thing about large number of atoms let say (N) number of atoms the split orbitals will be large and will form band of orbitals.
that is why , In solid crystal we can observe excising of valence band and conduction band and between of them region as the combination of forbidden regions of all atoms called band gap .We can definition the band gap in other word as the distance which the electrons must be crossed from the valence band to reach the conduction band.
image-5
band gap of metals, semiconductors and insulators
We can classify semiconductor materials from composition aspect to two type :
- Elemental include one type of atoms like materials in column IV silicon(Si) and Germanium(Ge)
- Compounds :
- Binary: tow element one from III and the other one from V column such as ZnS used in television screens
- Ternary: three element compounds such as GaAsP which use to made photodetectors has long-wave boundary of photosensitivity
- Quaternary four elements compounds such as InGaAsP used in lasers and LED
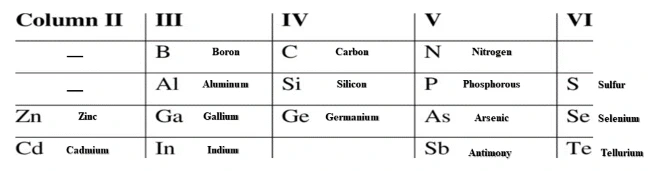
image-6
List of most used elements in semiconductor industry
Germanium was widely used in the early days of semiconductor developments for transistor and diodes, where nowadays Silicon is used for the majority of integrated circuits (ICs).
The compound semiconductors are widely used in high speed devices and devices required the emission or absorption light


